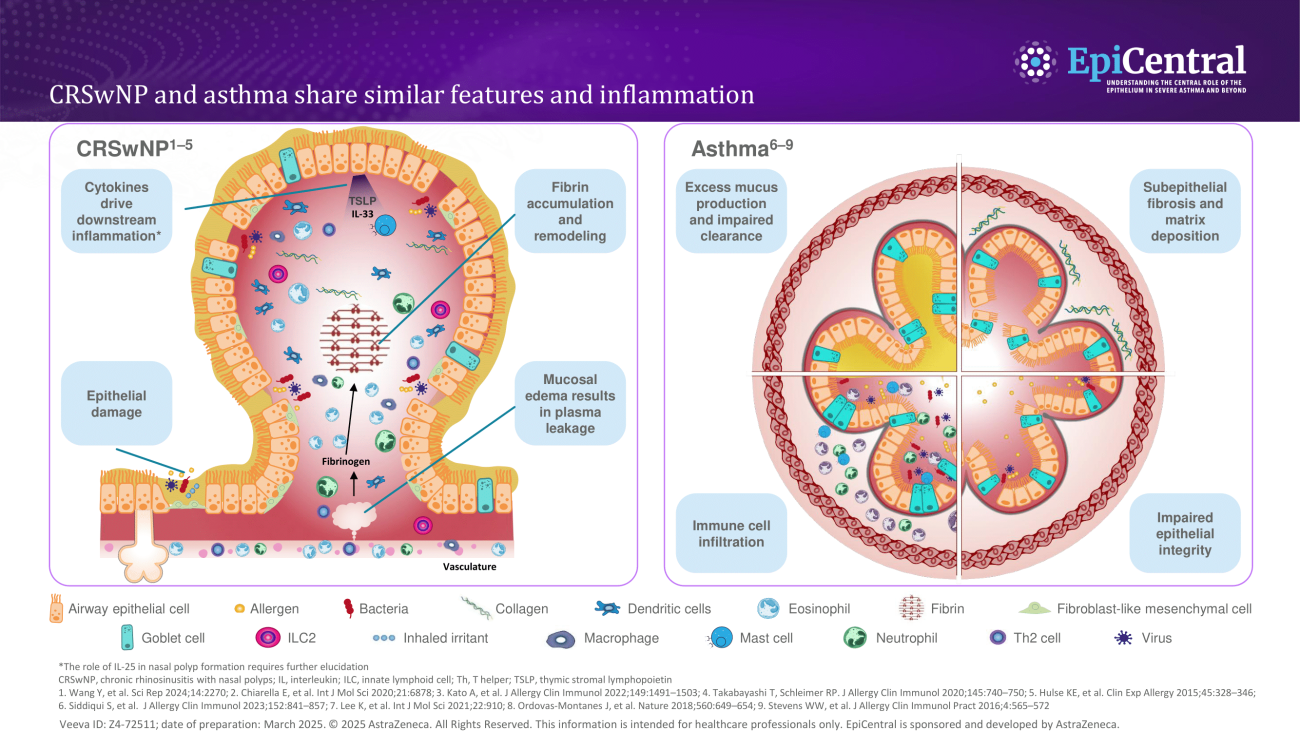
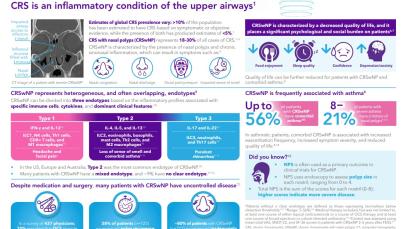
References
1. Wynne M, et al. Am J Rhinol Allergy. 2019;33:782–790;
2. Orlandi RR, et al. Int Forum Allergy Rhinol. 2021;11:213–739;
3. Sedaghat AR, et al. J Allergy Clin Immunol Pract. 2022;10:1395–1403;
4. Claeys N, et al. Front Allergy. 2021;2:761388;
5. Zhang M, et al. Int Immunopharmacol. 2023;121:110559;
6. Liao B, et al. Allergy. 2015;70:1169–1180;
7. Cho SH, et al. J Allergy Clin Immunol Pract. 2020;8:1505–1511;
8. Fokkens WJ, et al. Rhinology. 2020;58(Suppl. S29):1–464;
9. Benjamin MR, et al. J Allergy Clin Immunol Pract. 2019;7:1010–1016;
10. Soler ZM, et al. Laryngoscope. 2011;121:2672–2678;
11. Schneider S, et al. J Clin Med. 2020;9:925;
12. Kim J-Y, et al. JAMA Otolaryngol Head Neck Surg. 2019;145:313–319;
13. Tomoum MO, et al. Int Forum Allergy Rhinol. 2015;5:674–681;
14. Hopkins C, et al. Rhinology. 2015;53:18–24;
15. Hopkins C, et al. Rhinology. 2015;53:10–17;
16. Vennik J, et al. BMJ Open. 2019;9:e022644;
17. Hellings PW, et al. Rhinology. 2023;61:85–89;
18. De Corso E, et al. J Pers Med. 2022;12:897;
19. Price DB, et al. J Asthma Allergy. 2018;11:193–204;
20. Fokkens WJ, et al. Allergy. 2019;74:2312–2319;
21. van der Veen J, et al. Allergy. 2017;72:282–290;
22. DeConde AS, et al. Laryngoscope. 2017;127:550–555;
23. Chen S, et al. Curr Med Res Opin. 2020;36:1897–1911;
24. Starry A, et al. Allergy. 2022;77:2725–2736;
25. Peters A, et al. J Ann Allergy Asthma Immunol. 2024;133(Suppl.):S8 (Abstract D020);
26. Yan B, et al. J Allergy Clin Immunol. 2024;153:1206–1214;
27. Vroling AB, et al. Allergy. 2008;63:1110–1123;
28. Kato A, et al. J Allergy Clin Immunol. 2022;149:1491–1503;
29. Ha J-G, Cho H-J. Int J Mol Sci. 2023;24:14229;
30. Harkema JR, et al. Toxicol Pathol. 2006;34:252–269;
31. Tu Y, et al. Inflammation. 2021;44:1937–1948;
32. Gudis D, et al. Am J Rhinol Allergy. 2012;26:1–6;
33. Yan X, et al. Laryngoscope Investig Otolaryngol. 2020;5:992–1002;
34. Mullol J, et al. J Allergy Clin Immunol Pract. 2022;10:1434–1453;
35. Wang Y, et al. Sci Rep. 2024;14:2270;
36. Gong X, et al. Front Immunol. 2023;14:1238673;
37. Chan Y, et al. J Otolaryngol Head Neck Surg. 2023;52:50;
38. Staudacher AG, et al. Ann Allergy Asthma Immunol. 2020;124:318–325;
39. Stevens WW, et al. J Allergy Clin Immunol Pract. 2019;7:2812–2820;
40. Zhang Y, et al. J Allergy Clin Immunol. 2017;140:1230–1239;
41. Ryu G, et al. Precis Future Med. 2022;6:170–176;
42. Schleimer RP. Annu Rev Pathol. 2017;12:331–357;
43. Laidlaw TM, et al. J Allergy Clin Immunol Pract. 2021;9:1133–1141;
44. Gauvreau GM, et al. Allergy. 2023;78:402–417;
45. Duchesne M, et al. Front Immunol. 2022;13:975914;
46. Cho SH, et al. J Allergy Clin Immunol Pract. 2016;4:575–582;
47. Fokkens W, Reitsma S. Otolaryngol Clin North Am. 2023;56:1–10;
48. Lin DC, et al. Am J Rhinol Allergy. 2011;25:205–208;
49. Denlinger LC, et al. Am J Respir Crit Care Med. 2017;195:302–313;
50. Ek A, et al. Allergy. 2013;68:1314–1321;
51. Khan A, et al. Rhinology. 2019;57:32–42.